Key points
- All elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. are arranged in a chart called the periodic table.
- The arrangement of elements is based on their structure and properties.
- A Russian scientist named Dmitri Mendeleev produced one of the first practical periodic tables in the 19th century.
Video
Watch this video to learn about the development of the periodic tableA table which lists all of the chemical elements and arranges them in a way that is useful. It allows us to spot patterns and make predictions about other elements..
Cal: OK, friends. Chemistry time and in the studio with me I have super science school teacher Mrs Roberts, who's gonna show us how to make a massive fizzing volcano. We're gonna have sparks, lava exploding, aren't we Miss?
Mrs Roberts: Not quite. So, today we're going to look at the periodic table. That thing that you see behind your teacher on the wall in science lessons. It actually tells us a lot about the elements that make up the world around us.
Cal: Ok. So, what actually is the periodic table?
Mrs Roberts: At its simplest, the periodic table is all the different elements arranged together in a chart. A Russian scientist called Dmitri Mendeleev produced one of the first practical periodic tables in the nineteenth century. The modern periodic table is based closely on the ideas he used.
Cal: And how is it ordered?
Mrs Roberts: So, the elements are arranged in order of increasing atomic number. The horizontal rows are called periods and the vertical columns are called groups, so elements in the same group as similar to each other.
Cal: Ah, OK. Now that does make a bit more sense.
How are the elements in the periodic table ordered?
Elements are arranged in order of increasing atomic number.

The development of the periodic table
In the 1800s, scientists had discovered many new elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table., but no system existed to organise them. Scientists were trying to look for similarities in their properties to arrange them in a meaningful way.
Early attempts to organise the elements
John Newlands noticed that if the elements were ordered by atomic weightAtomic weight is a measure of how heavy an atom is., every eighth element had similar properties. For example, sodium is the eight places further on than lithium and reacts in a very similar way.
For example, lithium (Li), sodium (Na) and potassium (K) all have the same kind of reaction with water.
| Li | Lithium |
| Na | Sodium |
| K | Potassium |
Julius Meyer arranged elements by atomic weight and by the number of atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. the element usually combined with.
The group of elements below were grouped together because they tended to combine with two other atoms. For example, one oxygen atom combines with two hydrogen atoms to make water (H₂O).
| O | Oxygen |
| S | Sulfur |
| Se | Selenium |
| Te | Tellurium |
Mendeleev’s periodic table

A Russian scientist called Dmitri Mendeleev produced one of the first practical periodic tables in the 19th century.
The modern periodic table is based closely on the ideas he used.
These ideas were:
- The elements are arranged in order of increasing atomic mass
- The horizontal rows are called periods
- The vertical columns are called groups
- Elements in the same group are similar to each other
Mendeleev's ideas are still in use today. However, the modern periodic table arranges the elements by increasing atomic number, rather than atomic mass.

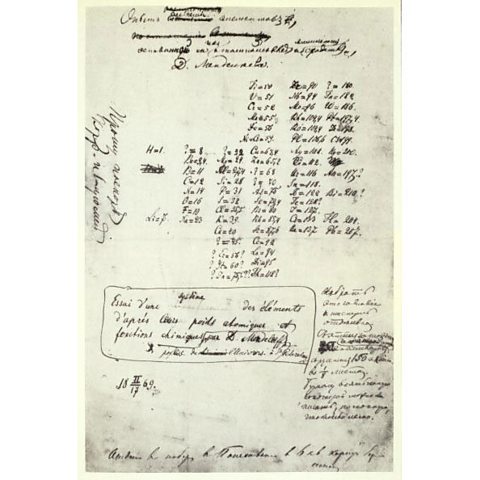
How did Mendeleev produce the periodic table?
Mendeleev wrote the names of elements onto cards to help him look for patterns. He put the cards in order of lightest to the heaviest element and made groups based on the chemical propertyThe way an element or compound reacts with other chemical substances. and physical propertyA property of an element or compound which can be directly observed or measured. For example, melting point, electrical conductivity, appearance at room temperature. of each element.
Mendeleev recognised that not all elements had been discovered yet. He left gaps in his table to place elements that scientists didn't yet know.
By looking at the physical properties and chemical properties of elements next to gaps, he could even predict the properties of these undiscovered elements.

These correct predictions increased the confidence in Mendeleev's periodic table.

Did you know?
Mendeleev’s work is the basis of the modern periodic table we use today.
He is often referred to as the 'father of the periodic table', but he didn’t achieve a Nobel Prize for his work.

Why did Mendeleev leave gaps in his original periodic table?
Because he recognised that not all elements had been discovered.
During Mendeleev’s time there were around only 65 known elements. Today we know there are 118 elements, all which can be found on the modern periodic table.
Working scientifically

The periodic table is a good example of how scientific ideas develop over time.
Mendeleev left gaps in his table when he drew it up in 1869, using his insights to predict that scientists would discover more elements. Since the 19th century, more elements have been discovered that fit these gaps, further confirming that Mendeleev’s understanding was correct.

The modern periodic table has no gaps and has extended beyond what Mendeleev predicted. There were 64 elements when Mendeleev was working on his table and he predicted 4 more. Now the periodic table has 118 elements.

Did you know?
The most recent element to be discovered is Tennesine which was made in a laboratory in 2009.
This element only exists for fractions of a second, because it is unstable meaning it breaks apart very quickly.

Test your knowledge
Quiz
Working safely in the lab
Find out how to spot risks, hazards and understand hazard symbols

More on Periodic table
Find out more by working through a topic
- count3 of 6
- count4 of 6

- count5 of 6

- count6 of 6
